
請用 Wechat 掃描台灣精品QRcode 或 搜尋ID: TaiwanExcellence

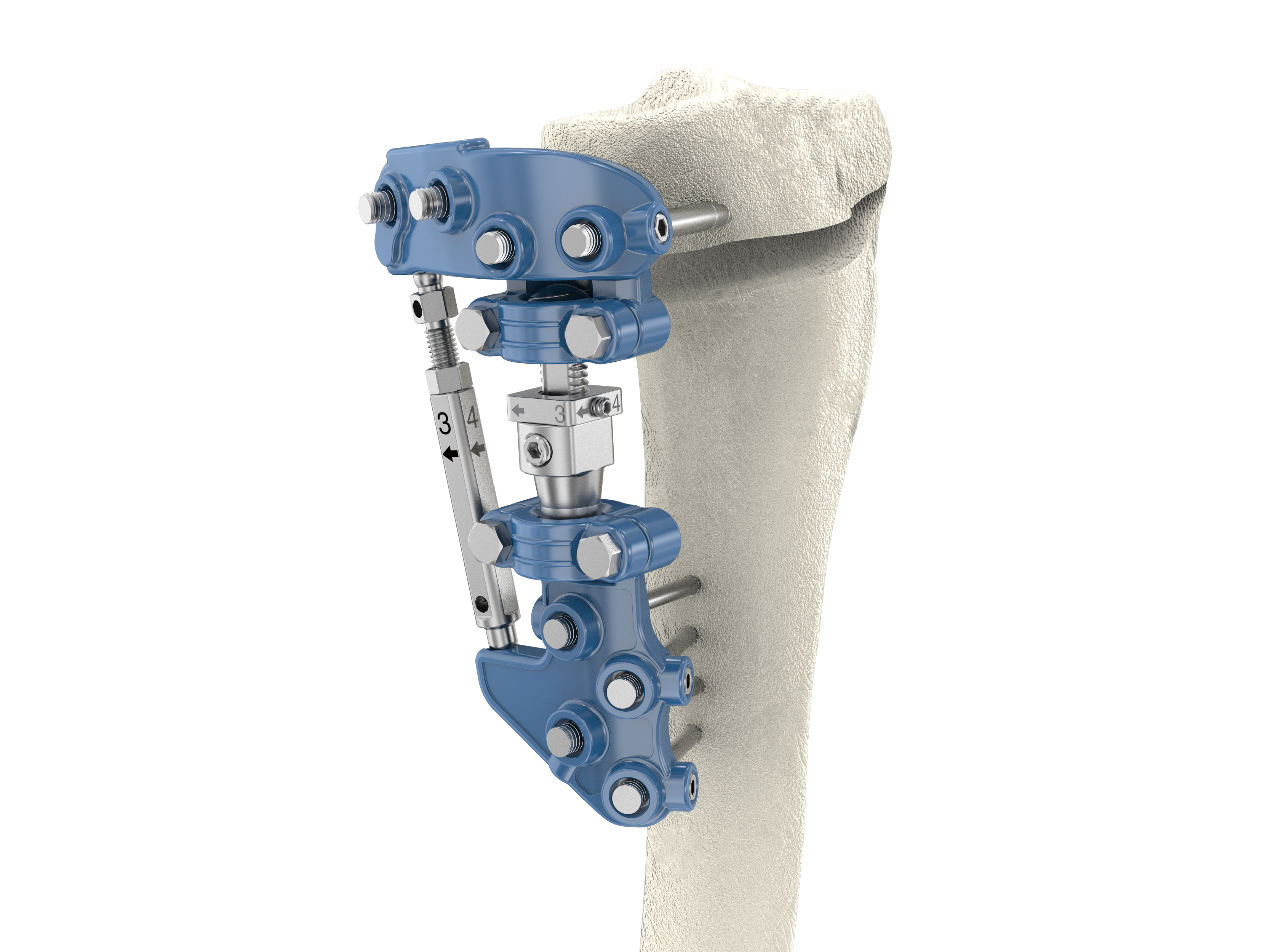
“Paonan” PK HTO Correction System is an external fixator for treating medial knee osteoarthritis through self-healing to delay joint replacement and has been approved by the FDA and TFDA. The system provides a specially designed instrument to ensure the safety and precision of surgical procedures. The advantages of this system are:
1. Low profile anatomical shape design which is more convenient for a patient’s daily life activities.
2. Minimally invasive surgery offers smaller incisions and the patient has a shorter hospital stay.
3. Application of distraction osteogenesis theory and Wolff’s law in product design to achieve shorter bone healing time and earlier full weight bearing after surgery.
4. Correction is more precise through phased external adjustment postoperatively.
5. Pins can be removed in phases to provide bone stimulation.
6. Pin is easily removed, if pin site infection is incurred.
7. Secondary surgery for removal of implant is unnecessary and easy to convert from HTO to TKA.
About Paonan Biotech
Paonan Biotech, founded in 1985, dedicated in long-term spinal implant and external fixation system development as a professional medical device manufacturer. Founder Mr. Benny Yeh developed the first spinal fixation system Rod Plate System successfully as the first Taiwan independent research and development product. Further innovated products, including AF, RF, and SRS, has became the earliest wide spreading spinal fixation and spondylolisthesis reduction system. Over 30 years, Paonan seeing “Minimally Invasive, Simplified Surgical Procedures, Dynamic Fusion, and Enhance Post-operative Outcome” as objective. Over 100 external fixation and spinal implant products were developed and made in Taiwan. Taiwanese are now able to enjoy high quality spinal medical device which is keeping pace with global spinal implants manufacturers. So far, our products have been marketed to over 30 countries from Asia to America and Europe.
Paonan Biotech was founded in 1985. Founder Mr. Benny Yeh has 35 years’ experience in orthopedic and spine medical product developing and marketing. Paonan R&D follow the spirit of the founder, seeing “Minimally Invasive, Simplified Surgical Procedures, Dynamic Fusion, and Enhance Post-operative Outcome” as objective. Paonan and Biomech are used in different countries to expand in/external market. Now, our products have been marketed to more than 30 countries in Asia, America, and Europe. Paonan expanded oversea market successfully while 3 products winning world’s 50 important spinal implant in 2014. 2018, Paonan received SBIR approval by Ministry of Economic Affairs. NEST, the brand new lumbar fusion cage made by unique 3D printing technology, set milestone in orthopedic history as the first 3D printing lumbar cage approved by TFDA and the first in Asia approved by FDA.